Abstract
Acute T-cell lymphoblastic leukemia (T-ALL) is an aggressive hematologic malignancy in children and young adults. When compared to B-ALL, T-ALL has a higher rate of induction failure as well as relapse, with very poor overall survival in both scenarios. T-ALL has not benefitted from the same immune-mediated therapies (e.g., anti-CD19 CAR-T cells) and poses an ongoing treatment challenge as well as an unmet therapeutic need.
Here, we demonstrate that inhibition of dihydroorotate dehydrogenase (DHODH), an enzyme that converts dihydroorotate to orotate as part of the de novo synthesis of uridine, has a robust anti-leukemia effect in both in vitro and in vivo models of T-ALL. DHODH is ubiquitously-expressed, and inhibition of DHODH (DHODHi) leads to rapid depletion of pyrimidine ribo- and deoxyribonucleotides. A cell's ability to tolerate periods of pyrimidine starvation is dependent on a variety of alternative salvage pathways and is not well-understood. Clinical grade DHODH inhibitors are currently under investigation in trials assessing their effect in myeloid malignancies, where they are also known to have a pre-clinical anti-leukemic effect.
We sought to understand the mechanism underlying the sensitivity of T-lymphoblasts to DHODHi, and to develop new DHODHi therapeutic combinations for patients with T-ALL. It is known that the intracellular concentration of nucleotides is high in activated T-lymphocytes, underlying the fact that two low-potency DHODHi's are approved for the treatment of autoimmune T-cell diseases (e.g., teriflunomide for multiple sclerosis and leflunomide for rheumatoid arthritis). We hypothesized that T-cells may have a particular addiction to de novo nucleotide synthesis and that this can be exploited as a vulnerability by the small molecule inhibition of DHODH.
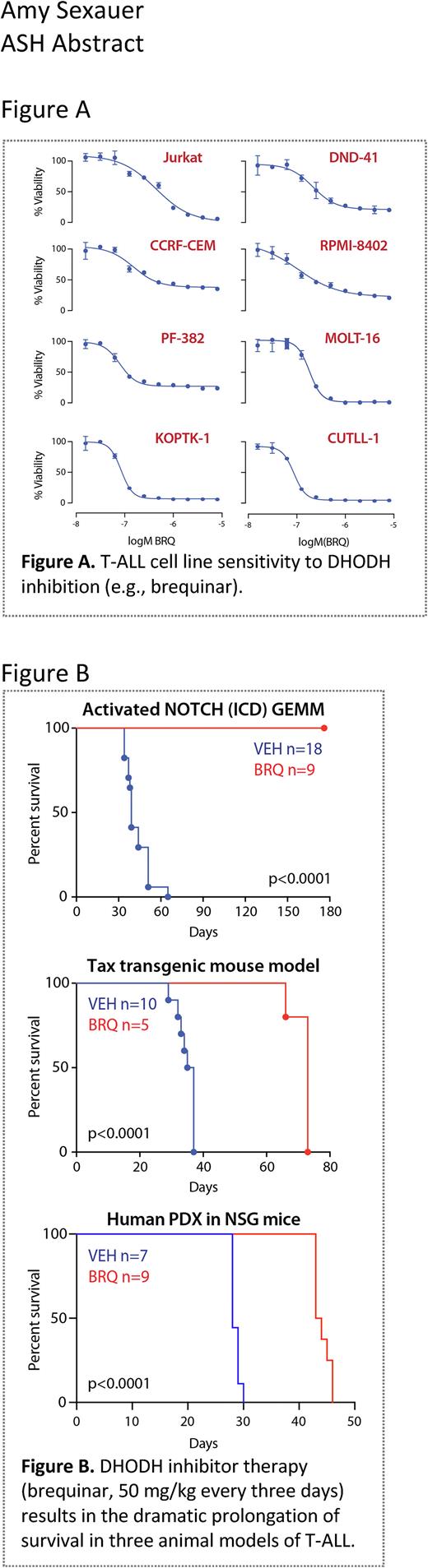
We demonstrated that T-ALL is highly sensitive to both chemical and genetic DHODHi using a variety of in vitro models (Figure A). Furthermore, we show that T-lymphoblasts respond to DHODHi by undergoing rapid cell cycle arrest and apoptosis. Additionally, we quantified the changes in intracellular nucleotides using PCA extraction and high-performance liquid chromatography (HPLC). The abundance of UTP, CTP, ATP, and GTP were determined across a panel of T-ALL cell lines at baseline and following treatment with a small molecular inhibitor of DHODH. The depletion of intracellular pyrimidines is rapid, falling to ~30% of baseline at 6 hours and confirming that the extracellular salvage is not able to meet the nucleotide demands of these cells even in the presence of complete media. Of note, the concentration of ATP and GTP are unaffected, confirming the specificity of DHODH on de novo pyrimidine synthesis as well as the viability of the cells over the 24-hour treatment.
In several in vivo T-ALL models, we demonstrated that DHODH inhibition (specifically using the small molecule inhibitor brequinar) led to rapid, robust disease response (Figure B). These single agent treatment studies (brequinar, 50 mg/kg given every 3-days) were initiated between day 7 - 14 when the leukemias had engrafted and were established with detectable disease in the bone marrow. Three model systems were used: (1) a genetically engineered mouse model (GEMM) of immature T-ALL in which an activated intracellular NOTCH is expressed by retroviral transduction, (2) a transgenic mouse model of mature T-cell leukemia/lymphoma driven by the HTLV1 protein Tax, and (3) a human PDX from a young man with relapsed/refractory T-ALL. DHODH inhibitor therapy was effective in all three models, though clearly and most dramatically prolonged the survival in the GEMM model, driven by dysregulated NOTCH.
We are now focused on elucidating the mechanisms by which T-lymphoblasts are so incredibly sensitive to inhibition of DHODH. New data suggests that changes in mitochondrial membrane potential and mitochondrial mass following exposure to DHODHi may offer additional insights into this sensitivity. We are also studying DHODHi in combination with other approved and experimental agents toward the goal of a new therapeutic combination that is safe, well-tolerated, and efficacious for this patient population.
Disclosures
Gandhi:Sunesis: Honoraria, Research Funding; Pharmacyclics: Research Funding; Abbvie: Research Funding; Clear Creek Bio: Consultancy, Research Funding; Dava Oncology: Honoraria; LOXO: Research Funding. Stegmaier:AstraZeneca: Consultancy; Auron Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; KronosBio: Consultancy, Research Funding. Sykes:Clear Creek Bio: Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal